Embark on a scientific journey with the Colligative Properties Gizmo Answer Key, a comprehensive guide that unlocks the mysteries of solutions. Delving into the fundamental principles of colligative properties, this resource empowers you with the knowledge to understand how these properties shape the behavior of mixtures, impacting diverse fields from chemistry to medicine.
Discover the profound implications of colligative properties, unraveling their applications in everyday life. From the icy roads of winter to the delicate balance of intravenous fluids, explore the practical significance of these properties that govern the behavior of solutions.
Colligative Properties
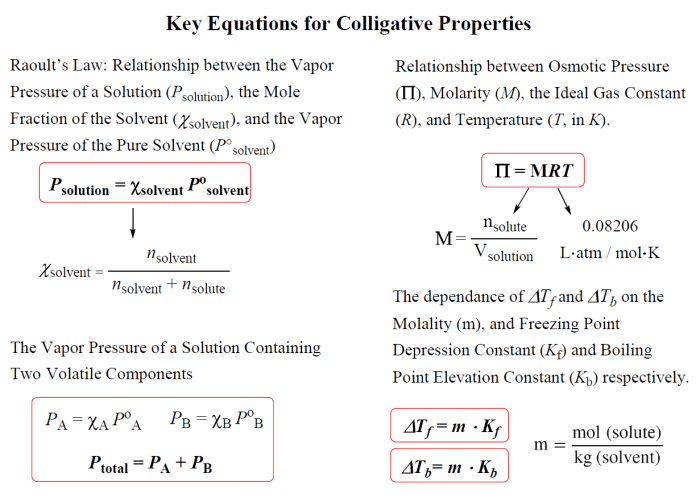
Colligative properties are physical properties of solutions that depend only on the concentration of solute particles, not on their chemical nature. The four colligative properties are freezing point depression, boiling point elevation, osmotic pressure, and vapor pressure lowering.
Freezing point depression is the decrease in the freezing point of a solvent when a solute is added. Boiling point elevation is the increase in the boiling point of a solvent when a solute is added. Osmotic pressure is the pressure that must be applied to a solution to prevent the passage of solvent molecules from a pure solvent into the solution.
Vapor pressure lowering is the decrease in the vapor pressure of a solvent when a solute is added.
Colligative properties are used in everyday life in a variety of applications. For example, antifreeze is added to car engines to prevent the water in the engine from freezing. Salt is added to roads in winter to melt ice and snow.
Intravenous fluids are used in medicine to replace lost fluids and electrolytes.
Gizmo Answer Key
The Gizmo answer key for the Colligative Properties simulation provides a step-by-step guide to solving the problems in the simulation. The answer key includes the following information:
- A brief overview of the concepts of freezing point depression, boiling point elevation, osmotic pressure, and vapor pressure lowering.
- The equations used to calculate each colligative property.
- Instructions on how to use the Gizmo to calculate colligative properties.
The Gizmo answer key is a valuable resource for students who are learning about colligative properties.
Applications of Colligative Properties

Colligative properties have a wide range of applications in various fields, including chemistry, biology, and medicine.
In chemistry, colligative properties are used to determine the molar mass of unknown substances. By measuring the freezing point depression or boiling point elevation of a solution, it is possible to calculate the concentration of the solute and hence its molar mass.
In biology, colligative properties are used to study the behavior of solutions. For example, osmotic pressure is used to measure the water potential of cells. Water potential is a measure of the tendency of water to move into or out of a cell.
It is important for cells to maintain a proper water potential in order to function properly.
In medicine, colligative properties are used to develop intravenous fluids. Intravenous fluids are used to replace lost fluids and electrolytes in patients who are unable to drink or eat. The colligative properties of intravenous fluids are carefully controlled to ensure that they are isotonic with the patient’s blood.
Real-World Examples: Colligative Properties Gizmo Answer Key
Colligative properties are used in a variety of everyday applications.
- Antifreeze is added to car engines to prevent the water in the engine from freezing. Antifreeze lowers the freezing point of water, which prevents the engine from freezing in cold weather.
- Salt is added to roads in winter to melt ice and snow. Salt lowers the freezing point of water, which causes the ice and snow to melt.
- Intravenous fluids are used in medicine to replace lost fluids and electrolytes. Intravenous fluids are isotonic with the patient’s blood, which means that they have the same osmotic pressure as the patient’s blood. This prevents the patient’s cells from shrinking or swelling.
Colligative properties are important in a variety of applications, from everyday life to medicine. They are a fundamental part of chemistry and are used to study the behavior of solutions.
User Queries
What are colligative properties?
Colligative properties are properties of solutions that depend solely on the concentration of solute particles, regardless of their identity.
How can I use the Gizmo to calculate colligative properties?
The Gizmo allows you to manipulate various parameters, such as solute concentration and temperature, to observe the effects on colligative properties.
What are some real-world applications of colligative properties?
Colligative properties find applications in diverse fields, including determining molar mass, studying solution behavior, and designing antifreeze and intravenous fluids.
