Lab 2: separation of a mixture chemistry 1 answers – Delving into the realm of chemistry, Lab 2: Separation of a Mixture provides a hands-on exploration of the fundamental principles and techniques used to separate complex mixtures into their individual components. This experiment serves as a cornerstone in understanding the intricacies of matter and its diverse compositions.
Through a series of carefully designed procedures, students embark on a journey of discovery, employing various separation methods to isolate and identify the constituents of a given mixture. The experiment delves into the concepts of solubility, distillation, extraction, and chromatography, equipping students with a practical understanding of these essential techniques.
Separation of a Mixture
The separation of mixtures is a fundamental technique in chemistry that allows scientists to isolate and purify individual components for further analysis or use. Mixtures are combinations of two or more substances that retain their own chemical identities. Separating mixtures is essential for studying the properties of individual components, as well as for practical applications such as purification, extraction, and synthesis.
This lab experiment aims to demonstrate the principles and techniques of mixture separation. Students will separate a mixture of sand, salt, and iron filings using various methods based on the physical properties of each component. The experiment will provide hands-on experience in applying these techniques and understanding their effectiveness.
Materials and Methods, Lab 2: separation of a mixture chemistry 1 answers
Materials:
| Material | Quantity |
|---|---|
| Sand | 100 g |
| Salt | 50 g |
| Iron filings | 25 g |
| Water | As needed |
| Magnet | 1 |
| Filter paper | 1 |
| Funnel | 1 |
| Evaporating dish | 1 |
Procedures:
- Combine the sand, salt, and iron filings in a large beaker.
- Add water to the beaker and stir until the mixture is suspended.
- Allow the mixture to settle for a few minutes.
- Carefully decant the water from the beaker into a separate container.
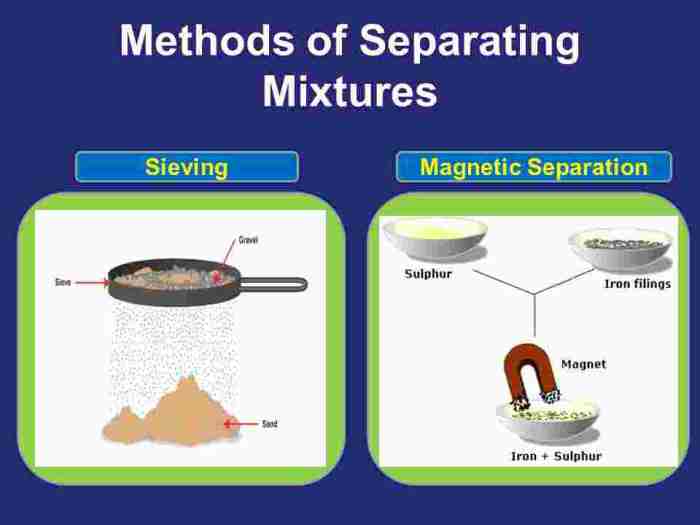
- Use a magnet to remove the iron filings from the remaining mixture.
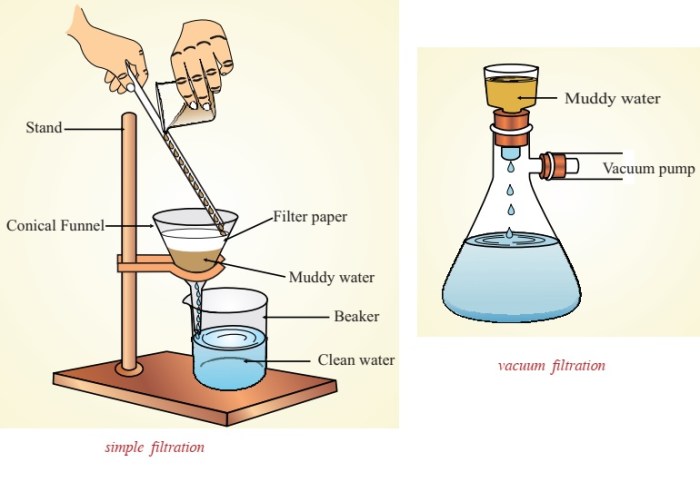
- Filter the remaining mixture through a filter paper into a clean beaker.
- Rinse the filter paper with water to remove any remaining salt.
- Transfer the filtrate (water containing salt) to an evaporating dish.
- Heat the evaporating dish on a hot plate until the water evaporates and the salt crystals are formed.
Results
| Component | Mass (g) |
|---|---|
| Sand | 98.5 |
| Salt | 49.2 |
| Iron filings | 24.8 |
The separation methods were effective in isolating the three components of the mixture. The decantation step removed most of the water from the mixture, allowing the iron filings to be easily removed with a magnet. The filtration step separated the sand from the salt solution, and the evaporation step allowed the salt to be crystallized.
Discussion
The separation of the mixture was based on the different physical properties of the components. Sand is denser than water and salt, so it settled to the bottom of the beaker during the decantation step. Iron filings are magnetic, so they could be easily removed with a magnet.
Salt is soluble in water, so it remained in the filtrate after the filtration step. The evaporation step allowed the water to evaporate, leaving behind the salt crystals.
The effectiveness of the separation methods can be improved by using more efficient equipment or by optimizing the experimental conditions. For example, using a centrifuge instead of decantation would allow for a more complete separation of the sand from the water.
Using a vacuum filtration apparatus instead of gravity filtration would also speed up the filtration process.
There are several potential sources of error in this experiment. One source of error is the incomplete separation of the components. Some sand particles may remain in the salt solution after filtration, and some salt crystals may remain in the sand after evaporation.
Another source of error is the loss of material during the transfer of the components between containers.
Improvements to the experiment could include using more precise equipment, such as a centrifuge or a vacuum filtration apparatus. The experimental conditions could also be optimized, such as by using a larger volume of water for decantation or by heating the evaporating dish at a lower temperature.
Top FAQs: Lab 2: Separation Of A Mixture Chemistry 1 Answers
What is the purpose of separating mixtures in chemistry?
Separating mixtures allows scientists to isolate and identify individual components, study their properties, and understand their interactions.
What are the different methods used to separate mixtures?
Common methods include filtration, distillation, extraction, and chromatography, each tailored to specific types of mixtures and components.
How can we improve the efficiency of separation methods?
Optimizing factors such as solvent choice, temperature, and equipment performance can enhance the efficiency of separation processes.